POLYMER GUIDEWIRE WITH HYDROPHILIC COATING, ENTER
1. DEVICE DESCRIPTION
The polymer guidewire with a hydrophilic coating Enter consists of a nickel-titanium alloy (nitinol) core wire coated with radiopaque polyurethane. The distal part of the guidewire may have a straight or angled tip.
-
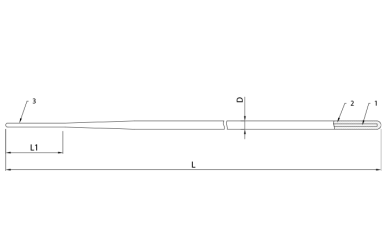
Fig. 1. Guidewire Enter – straight type
- Nitinol core
- Radiopaque coating
- Distal tip
L - Guidewire legth
L1 - Distal flexible part length
D - Guidewire diameter
-
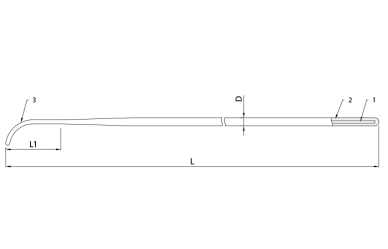
Fig. 2. Guidewire Enter – angled type
- Nitinol core
- Radiopaque coating
- Distal tip
L - Guidewire length
L1 - Distal flexible part length
D - Guidewire diameter
Table 1. Device parameters
Core material
Nitinol (nickel-titanium alloy)
Radiopaque coating
Polyurethane containing barium sulfate
Hydrophilic coating
Yes
Guidewire diameters
0.025’’; 0.032’’; 0.035’’; 0.038’’
Guidewire length
50 cm – 260 cm
Distal flexible part length
30 mm
Guidewire stiffness
Medium stiff for 0.025’’ and 0.032”;
medium stiff , stiff or floppy for 0.035’’ and 0.038’’
Distal tip
Straight or angled
2. PRODUCT RANGE
Table 2. Available device versions
Guidewire
diameter [inch]
Guidewire
stiffness
Guidewire lengths
[cm]
50
80
120
150
180
200
260
0.025
Medium stiff
Ö
Ö
Ö
Ö
Ö
Ö
Ö
0.032
Medium stiff
Ö
Ö
Ö
Ö
Ö
Ö
Ö
0.035
Floppy
Ö
Ö
Ö
Ö
Ö
Ö
Ö
Medium stiff
Ö
Ö
Ö
Ö
Ö
Ö
Ö
Stiff
Ö
Ö
Ö
Ö
Ö
Ö
Ö
0.038
Floppy
Ö
Ö
Ö
Ö
Ö
Ö
Ö
Medium stiff
Ö
Ö
Ö
Ö
Ö
Ö
Ö
Stiff
Ö
Ö
Ö
Ö
Ö
Ö
Ö
Ö - offered
3. INTENDED PURPOSE / INDICATIONS
The polymer guidewire with hydrophilic coating Enter is intended for facilitating the placement of balloon dilatation catheters and other interventional devices during endovascular procedures within peripheral arteries.
3.1. CONTRAINDICATIONS
The use of the device is usually contraindicated in the following cases:
- any cases listed as contraindications in the instructions for use of the product with which the guidewire is used;
- patients with contraindication(s) for antiplatelet / anticoagulant treatment;
- fully occluded vessel;
- allergy to device materials (polyurethane, nitinol).
3.2. POTENTIAL ADVERSE EVENTS
Adverse events that may be associated with the use of this device include (in alphabetical order), but are not limited to:
- allergic reaction or hypersensitivity to administered anticoagulation or antiplatelet drugs, anesthesia, contrast agent or guidewire materials;
- cardiac arrhythmias;
- death;
- fever;
- guidewire entrapment / fracture;
- hypotension / hypertension;
- infection;
- lower limb artery complications:
- abrupt closure;
- dissection;
- embolism (caused by air, atherosclerotic plaque, thrombotic material or device element);
- perforation;
- spasm;
- thrombosis;
- nausea and vomiting;
- pain;
- palpitations
- dizziness
- syncope;
- renal insufficiency / failure;
- stroke / transient ischemic attack (TIA);
- vascular access complications that may require blood transfusion or vessel repair:
- bleeding (ecchymosis, hematoma, hemorrhage, retroperitoneal hemorrhage);
- embolism (caused by air, atherosclerotic plaque, thrombotic material or device element);
- peripheral ischemia;
- peripheral nerve injury;
- pseudoaneurysm, dissection, perforation, arteriovenous fistula.
Any serious incident that has occurred in relation to the device should be reported to the manufacturer at reklamacje@balton.pl and the competent authority of the country in which the user and/or patient is established.
3.3. INTENDED USER PROFILE
Intended users of this device are only physicians who have received appropriate training for endovascular procedures.
3.4. USE ENVIRONMENT
Use of this device is allowed only in healthcare facilities prepared for endovascular procedures.
3.5. PATIENT TARGET GROUP
The target group are patients requiring endovascular procedures in peripheral arteries. No known data restrict the use of this device in patients of particular gender or race. Before making a decision concerning procedure with the use of the guidewire, potential benefits and risks should be considered individually for every patient.
6. HOW SUPPLIED
6.1. CONTENT OF THE PACKAGING
One (1) guidewire in a protective hoop, in a pouch, packed with the Instructions for Use in a unit box.
6.2. STERILITY
This product is supplied sterilized with ethylene oxide gas in a pouch. Only the content of the pouch should be considered sterile. The device is only sterile if this packaging is not opened or damaged.
7. HANDLING AND STORAGE
Store at room temperature in a dry place, in the unit box, as supplied. Do not expose to temperatures outside the range: 10 ºC ÷ 30 ºC.
9. WARRANTY
If delivered product is damaged or has any other defects, please inform the manufacturer and keep the device with original packaging.
10. OPERATIONAL INSTRUCTIONS
Balton sp. z o.o. shall not be liable for any direct, incidental or consequential damages resulting from the misuse of this product.
- Select the version / size of the guidewire suitable for the device it is to be used with.
- Check the primary packaging (pouch) for possible damage and expiry date.
If there is a suspicion that sterility may be compromised and/or the expiry date has been exceeded, it must not be used.
- Open the pouch and take out the guidewire in the protective hoop.
- Flush the guidewire in the hoop with heparinized normal saline in order to activate the hydrophilic coating.
- Remove the guidewire from the hoop and check if the guidewire is not bent, kinked or damaged.
If there is a suspicion that the device is damaged, it must not be used.
- Straighten the guidewire tip with the provided straightener.
- Place the guidewire in a vascular access device and gently advance towards the desired position.
Do not use excessive force when inserting the guidewire. If any resistance is encountered during insertion, withdraw the guidewire partially, twist slightly and try to gently advance again.
Remember to leave a sufficiently long part of the guidewire outside the vessel for easier maneuvering and to prevent embolization by uncontrolled displacement of the guidewire in the vessel.
Do not withdraw the guidewire against the needle bevel (Fig. 3.) - it may damage and/or peel the polymer coating, as well as damage and/or shear off the tip of the guidewire.

Fig. 3. Damage of the polymer coating
- Remove the guidewire once after completing the procedures that required its use.
SYMBOLS GLOSSARY
-
CE mark
-
Medical device
-
Unique device identifier
-
Manufacturer
-
Date of manufacture
-
Use-by date
-
Catalogue number
-
Batch code
-
Consult instructions for use or consult electronic instructions for use
-
Do not use if package is damaged and consult instructions for use
-
Caution
-
Sterilized using ethylene oxide
-
Do not resterilize
-
Do not re-use
-
Single sterile barrier system
-
Single sterile barrier system with protective packaging outside
-
Non-pyrogenic
-
Keep away from sunlight
-
Fragile, handle with care
-
Temperature limit
-
Not made with natural rubber latex
-
n units per package
-
Keep dry
-
Recyclable packaging material
-
Guidewire length
-
Guidewire diameter
-
Guidewire stiffness - FLOPPY
-
Guidewire stiffness – MEDIUM STIFF
-
Guidewire stiffness - STIFF
-
Tip type – ANGLED
-
Tip type – STRAIGHT
1. DEVICE DESCRIPTION
The polymer guidewire with a hydrophilic coating Enter consists of a nickel-titanium alloy (nitinol) core wire coated with radiopaque polyurethane. The distal part of the guidewire may have a straight or angled tip.
-

Fig. 1. Guidewire Enter – straight type
- Nitinol core
- Radiopaque coating
- Distal tip
L - Guidewire legth
L1 - Distal flexible part length
D - Guidewire diameter
-

Fig. 2. Guidewire Enter – angled type
- Nitinol core
- Radiopaque coating
- Distal tip
L - Guidewire length
L1 - Distal flexible part length
D - Guidewire diameter
Table 1. Device parameters
| Core material | Nitinol (nickel-titanium alloy) |
|---|---|
| Radiopaque coating | Polyurethane containing barium sulfate |
| Hydrophilic coating | Yes |
| Guidewire diameters | 0.025’’; 0.032’’; 0.035’’; 0.038’’ |
| Guidewire length | 50 cm – 260 cm |
| Distal flexible part length | 30 mm |
| Guidewire stiffness | Medium stiff for 0.025’’ and 0.032”; medium stiff , stiff or floppy for 0.035’’ and 0.038’’ |
| Distal tip | Straight or angled |
2. PRODUCT RANGE
Table 2. Available device versions
| Guidewire diameter [inch] |
Guidewire stiffness |
Guidewire lengths [cm] |
||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 80 | 120 | 150 | 180 | 200 | 260 | ||
| 0.025 | Medium stiff | Ö | Ö | Ö | Ö | Ö | Ö | Ö |
| 0.032 | Medium stiff | Ö | Ö | Ö | Ö | Ö | Ö | Ö |
| 0.035 | Floppy | Ö | Ö | Ö | Ö | Ö | Ö | Ö |
| Medium stiff | Ö | Ö | Ö | Ö | Ö | Ö | Ö | |
| Stiff | Ö | Ö | Ö | Ö | Ö | Ö | Ö | |
| 0.038 | Floppy | Ö | Ö | Ö | Ö | Ö | Ö | Ö |
| Medium stiff | Ö | Ö | Ö | Ö | Ö | Ö | Ö | |
| Stiff | Ö | Ö | Ö | Ö | Ö | Ö | Ö | |
Ö - offered
3. INTENDED PURPOSE / INDICATIONS
The polymer guidewire with hydrophilic coating Enter is intended for facilitating the placement of balloon dilatation catheters and other interventional devices during endovascular procedures within peripheral arteries.
3.1. CONTRAINDICATIONS
The use of the device is usually contraindicated in the following cases:
- any cases listed as contraindications in the instructions for use of the product with which the guidewire is used;
- patients with contraindication(s) for antiplatelet / anticoagulant treatment;
- fully occluded vessel;
- allergy to device materials (polyurethane, nitinol).
3.2. POTENTIAL ADVERSE EVENTS
Adverse events that may be associated with the use of this device include (in alphabetical order), but are not limited to:
- allergic reaction or hypersensitivity to administered anticoagulation or antiplatelet drugs, anesthesia, contrast agent or guidewire materials;
- cardiac arrhythmias;
- death;
- fever;
- guidewire entrapment / fracture;
- hypotension / hypertension;
- infection;
- lower limb artery complications:
- abrupt closure;
- dissection;
- embolism (caused by air, atherosclerotic plaque, thrombotic material or device element);
- perforation;
- spasm;
- thrombosis;
- nausea and vomiting;
- pain;
- palpitations
- dizziness
- syncope;
- renal insufficiency / failure;
- stroke / transient ischemic attack (TIA);
- vascular access complications that may require blood transfusion or vessel repair:
- bleeding (ecchymosis, hematoma, hemorrhage, retroperitoneal hemorrhage);
- embolism (caused by air, atherosclerotic plaque, thrombotic material or device element);
- peripheral ischemia;
- peripheral nerve injury;
- pseudoaneurysm, dissection, perforation, arteriovenous fistula.
Any serious incident that has occurred in relation to the device should be reported to the manufacturer at reklamacje@balton.pl and the competent authority of the country in which the user and/or patient is established.
3.3. INTENDED USER PROFILE
Intended users of this device are only physicians who have received appropriate training for endovascular procedures.
3.4. USE ENVIRONMENT
Use of this device is allowed only in healthcare facilities prepared for endovascular procedures.
3.5. PATIENT TARGET GROUP
The target group are patients requiring endovascular procedures in peripheral arteries. No known data restrict the use of this device in patients of particular gender or race. Before making a decision concerning procedure with the use of the guidewire, potential benefits and risks should be considered individually for every patient.
6. HOW SUPPLIED
6.1. CONTENT OF THE PACKAGING
One (1) guidewire in a protective hoop, in a pouch, packed with the Instructions for Use in a unit box.
6.2. STERILITY
This product is supplied sterilized with ethylene oxide gas in a pouch. Only the content of the pouch should be considered sterile. The device is only sterile if this packaging is not opened or damaged.
7. HANDLING AND STORAGE
Store at room temperature in a dry place, in the unit box, as supplied. Do not expose to temperatures outside the range: 10 ºC ÷ 30 ºC.
9. WARRANTY
If delivered product is damaged or has any other defects, please inform the manufacturer and keep the device with original packaging.
10. OPERATIONAL INSTRUCTIONS
Balton sp. z o.o. shall not be liable for any direct, incidental or consequential damages resulting from the misuse of this product.
- Select the version / size of the guidewire suitable for the device it is to be used with.
- Check the primary packaging (pouch) for possible damage and expiry date.
- Open the pouch and take out the guidewire in the protective hoop.
- Flush the guidewire in the hoop with heparinized normal saline in order to activate the hydrophilic coating.
- Remove the guidewire from the hoop and check if the guidewire is not bent, kinked or damaged.
- Straighten the guidewire tip with the provided straightener.
- Place the guidewire in a vascular access device and gently advance towards the desired position.
Do not use excessive force when inserting the guidewire. If any resistance is encountered during insertion, withdraw the guidewire partially, twist slightly and try to gently advance again.
Remember to leave a sufficiently long part of the guidewire outside the vessel for easier maneuvering and to prevent embolization by uncontrolled displacement of the guidewire in the vessel.
Do not withdraw the guidewire against the needle bevel (Fig. 3.) - it may damage and/or peel the polymer coating, as well as damage and/or shear off the tip of the guidewire.

Fig. 3. Damage of the polymer coating
- Remove the guidewire once after completing the procedures that required its use.
SYMBOLS GLOSSARY
-
CE mark
-
Medical device
-
Unique device identifier
-
Manufacturer
-
Date of manufacture
-
Use-by date
-
Catalogue number
-
Batch code
-
Consult instructions for use or consult electronic instructions for use
-
Do not use if package is damaged and consult instructions for use
-
Caution
-
Sterilized using ethylene oxide
-
Do not resterilize
-
Do not re-use
-
Single sterile barrier system
-
Single sterile barrier system with protective packaging outside
-
Non-pyrogenic
-
Keep away from sunlight
-
Fragile, handle with care
-
Temperature limit
-
Not made with natural rubber latex
-
n units per package
-
Keep dry
-
Recyclable packaging material
-
Guidewire length
-
Guidewire diameter
-
Guidewire stiffness - FLOPPY
-
Guidewire stiffness – MEDIUM STIFF
-
Guidewire stiffness - STIFF
-
Tip type – ANGLED
-
Tip type – STRAIGHT